Why in News:Recent discussions and policy proposals are focused on streamlining the approval process for biosimilars by reducing the need for expensive animal and clinical trials. .
What is a Biosimilar?
- A biosimilar is a biologic medical product that is highly similar to an original, patented biologic product (also called the “innovator” product).
Types of Drugs: Small Molecules vs Biologics
Small Molecule Drugs:
- Chemically simple, fixed structures.
- Example: Disprin (~180 daltons).
- Stable structure throughout use.
Biologics:
- Large, complex molecules produced in biological systems.
- Examples: Insulin (~5,800 daltons), monoclonal antibody Remicade (~150,000 daltons).
- Slight structural variations may occur but usually don’t affect efficacy or safety.
Patent Protection and Market Dynamics
Small Molecule Drugs:
- When first developed, these drugs receive patent protection, granting exclusivity to the innovator company for several years.
- This exclusivity allows high pricing due to lack of competition.
- After patent expiry, other companies produce generics — identical copies that are cheaper because they avoid costly research and marketing.
- Example: Hepatitis C drug Sovaldi dropped from $84,000 to $1,000 after Indian generic firms entered the market.
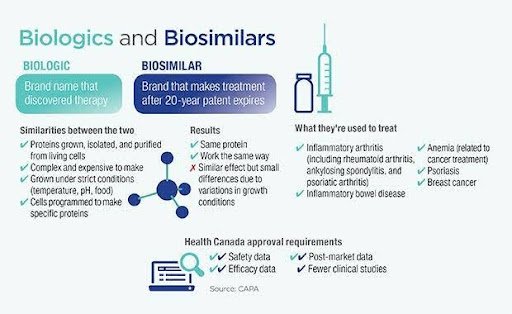
Biologics and Biosimilars:
- Biologics are also patent-protected but are complex and produced by living systems, so exact copies are impossible.
- Competing products made post-patent are called biosimilars, not generics, due to slight structural differences.
- Proving biosimilarity is more challenging and expensive than for generics.
Differences Between Biosimilars and Generics
Product Type:
- Biosimilars are equivalents of complex biological products; generics are copies of simple chemical drugs (APIs).
Manufacturing:
- Biosimilars are produced by engineering living organisms with inherent variations; generics are exact chemical copies.
Complexity:
- Biosimilars have complex structures with potential variations; generics have simple, well-defined structures.
Regulatory Approval:
- Biosimilars require more rigorous testing to prove similarity; generics need simpler bioequivalence data.
Prospects of Biosimilars
- The growing biologics market for cancer, diabetes, and autoimmune diseases boosts biosimilar demand globally.
- Indian pharma companies are investing to gain early market advantage; Zydus Cadila led with the first Adalimumab biosimilar in 2014.
- Biosimilars reduce high biologics’ costs, improving patient access, especially in developing countries.
- Patent expiries of biologics open opportunities for biosimilars to fill treatment gaps.
- Rising non-communicable diseases (cancer, asthma, arthritis) increase the need for affordable biosimilars.
- Biosimilars promote pharma innovation, growth, and profitability.
Global debate on simplifying Biosimilar Approval
Countries (US, UK, Europe) are working to simplify approval processes using advanced analytical methods:
- UK has eliminated mandatory animal trials.
- US plans to replace animal tests with human-relevant methods (e.g., organoids).
- India has not fully updated its guidelines but proposes waiving animal studies case-by-case.
- Clinical trials remain expensive but are required only in some cases in countries like the UK.
Why Simplified Approval Processes
- Cost Reduction: Complex clinical trials and animal testing significantly increase biosimilar costs, limiting affordability.
- Improved Patient Access: Lower costs from simplified testing can make lifesaving biologic treatments accessible to a larger population.
- Encouraging Competition: Streamlining regulatory requirements encourages more manufacturers to enter the market, fostering competition and innovation.
- Technological Advances: Modern analytical methods can reliably establish biosimilarity without extensive animal or clinical testing.
Way Forward: Affordable and Effective Biosimilars
- Need to balance cost reduction with maintaining efficacy and safety.
- Simplifying approval processes using modern techniques can reduce costs.
- Greater availability of affordable biosimilars will expand treatment options and improve patient access.
- India can adopt international best practices to streamline regulations and encourage domestic biosimilar production.
Conclusion
Biosimilars hold great promise to lower healthcare costs and increase drug accessibility. Regulatory reforms that embrace scientific advancements will be key to unlocking this potential.
UPSC Relevance:
GS Paper 2 (Governance and Health Policies)
GS Paper 3 (Science and Technology, Health)
UPSC Mains Practice Question
Q:“The rise of biosimilars presents a significant opportunity to improve healthcare accessibility and affordability in India. Discuss the challenges and prospects associated with the development and regulation of biosimilars in India.”
